Marine salts – introduction
Having in mind the controversy that the previous test aroused I defended myself for quite some time before making the new approach to the subject. However, I have to admit that for the past two years I regularly received emails asking for further tests. In marine aquaculture, two years is a long period of time, therefore I finally decided to re-measure using popular salts.
I was primarily interested in the changes of salt’s parameters that I have studied before as well as salt’s parameters that are recent in the market. Probably I won’t satisfy everyone again, but unfortunately a number of aquarium salts available on the market far exceeds my logistical and financial means, so believe me that the test of thirteen salts is the absolute maximum of my organizational capabilities. The test itself is almost twice as large as the previous test, and is twice as effective. This gives a huge amount of data to elaborate, which unfortunately is very laborious, considering that I do it all by myself.
I hope this test will not cause as much negative backlash as the previous one. Moreover, I wanted to point out that I’m not affiliated with any company selling marine salts for aquaristics. I have no commission whatsoever from selling any product of any brand taking part in the test. I assure you that despite the unquestionable curiosity of the results, I do not care that certain salt “won” this test. I deliberately skipped the salt I personally use, and consider one of the better ones on the market, so as not to be blamed for bias.
This text is by no means a purely scientific study, rather an objective analysis of a subject that, due to my experience, is enriched with subjective elements. As we touch on the topic of direct comparison of different products, I wanted to ensure that I made every effort to ensure that the information provided was accurate and consistent with the results. The aquarium salt test will not determine the winner or loser; Its task is to give you the information you need to make a personal decision.
The test itself is based on similar principles and is developed according to a similar scheme as the previous test. I strongly encourage you to read the previous material (http://reefhub.pl/test-soli-drugie-starcie/) as the descriptive part is slightly reduced in order to reduce the volume of the article.
Marine salts – sponsors
This project is a big logistics and financial challenge, so I wanted to thank the companies that provided the test salts. For all readers of this article, consider yourself invited to shop in the following stores:


And for all the shops, in the following wholesalers:
Marine salts – start
Aquarium salt together with water forms the basis of the marine environment in the aquarium. It is thanks to salt, we can enjoy the marine hobby. Aquarium salt is the base of any supplements in the aquarium and is intended to make the marine animals we buy feel good and healthy in our aquarium. However, the topic is much more complex than many people might expect. Ideal would be to use the natural seawater (NSW) from tropical regions, but this being impossible, manufacturers had to create a mixture of different ingredients that, when dissolved in RODi water, gives a saline composition similar to NSW.
Believe me, however, that the topic is not at all simple. The salt producer must be aware of many factors, such as impurities, fragmentation, hygroscopicity, accuracy, homogeneity of components, let alone the stability of humidity and air temperature in the production areas. This is followed by the manufacturer’s approach to the topic of optimal brine parameters, which has a direct impact on its composition and price.
There are many chemical elements in the sea water – almost whole Mendeleev’s Table, but do we really need all of them while copying the NSW composition? Of course not. First of all, it is neither profitable nor necessary. The repetition of salt parameters is far more important – so that each bucket of the batch has the same composition of major macro and micronutrients
Marine salts – Candidates for the throne
As I wrote earlier, there are a lot of different sea salts on the market, from which I had to choose a few to test. The basic criterion was the universality on the Polish market. In the forum you gave a lot of suggestions and it soon became clear that another test with six or seven salts wouldn’t be enough. Thanks to our sponsors help, we have managed to gather 13 salts that took part in the test. Which were (in alphabetical order):
Aquaforest Probiotic Reef Salt (AFPB)
Aquaforest Reef Salt (AFRS)
Colombo (COLOMBO)
Fauna Marin (FM)
Instant Ocean (IO)
Kent (KENT)
Living Colors (LC)
Microbe-Lift Organic Active (MLOA – in certain graphs labelled as MB OA)
Microbe-Lift Premium Reef (MLPR – in certain graphs labelled as MB PR)
Preis Meersalz (PR)
Reef Crystals (RC)
Red Sea Coral Pro (RSCP)
Red Sea Salt (RSS – in certain graphs labelled as RS)
The Salt Seachem Reef Salt supposed to be in the test. However, I got information from the distributor that a new salt will enter the market, which will replace the current one. So it did not make sense to test the “old” one, and “new” was not available yet.
From the above-mentioned salts, Microbe-Lift and Colombo salts are relatively new on the Polish market. The rest are already well known in the Polish aquaristic society.
Marine salts – measurements, procedures and results
In fact, the test itself is not too different from the previous one. Using each salt I made a 10-liter salinity solution of 35ppt which was then subjected to tests that I could perform at home. Then I took samples that were sent to the laboratory for the measurement of 35 different elements.
Marine salts – efficiency
The purpose of this study was to determine the amount of salt needed to saline 10L of RODi water up to 35ppt. The salinity was measured using a Deltec telescope refractometer. During the tests one of them crashed and despite repeated calibration, he underestimated the result by 2ppt. Unfortunately, I noticed this only after more than half of the measurements, when I compared it to the second refractometer to confirm the results. Unfortunately, the damage has been done and I decided to finish the salinity measurement on the same refractometer and then convert all the results to 35ppt. In this way any measurement error was the same for all salts. Moreover the brine temperature during the stirring was maintained at 25C.
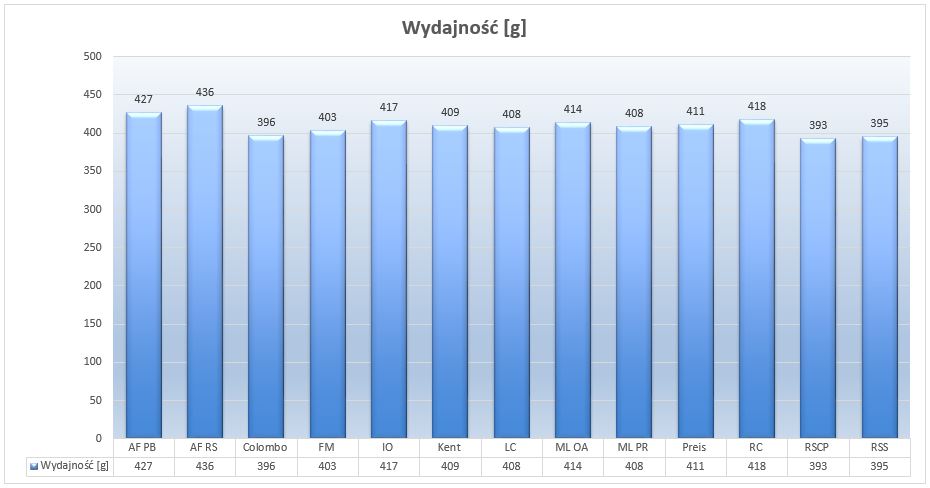
Picture 1- Performance of individual salts (in grams) per 10 liters of the solvent at salinity level of 35ppt
Let’s see what might affect the performance differences. Of course, what first comes to mind is the moisture of the individual salts. However, I think it is of little importance with the new packaging. I strongly suspect more hydration of the main ingredients of salt, eg calcium chloride. The more hydrated the compound, the more we need to use it to get the desired concentration. I already wrote about it in my previous article. Hydration takes place during crystallization of salt, water molecules get trapped in its crystal lattice. The more water molecules, the “amount of salt in salt” is less. For example, magnesium hexahydrate MgCl2x 6H2O contains as many as six water molecules, which together weigh more than the MgCl2 itself. In practice, this translates into salt efficiency. In order to obtain a one molar concentration of MgCl2 solution we have to pour about 95 g of anhydrous MgCl2 and about 203g of hexahydrate. 108g more to get the same concentration. This is the main reason why different salts have different efficiency. The second reason is the molar mass of the compounds used which are different in every salt. If we want to obtain a one-molar concentration of Mg2 ions + we have to add about 95 grams of anhydrous MgCl2 and, using magnesium sulphate, we have to add more than 120 grams of MgSO4.
You will probably ask why manufacturers do not use anhydrous substances. The answer is simple. In general, the more hydrated the compound, the less moisture it absorbs from the air, and therefore the salt is more durable and resistant to petrification.
Edit: I had some concerns about elevated performance of the Aquaforest salts. I am not saying whether they are good or bad, but I am going to investigate it in another project. Stay tuned.
Marine salts – pH after 45 minutes of mixing
After achieving the desired salinity, each brine was mixed for 45 minutes and then subjected to pH measurement. The measurement was made using an electronic AZ8686 measurer, which was checked several times a day in the control fluid.
PH is an important indicator of water quality and has a direct impact on the calcification processes. The perfect pH level in the aquarium should be in the range of 8.2 – 8.4, although the result above 8 can be considered satisfactory. PH results below 7.7 are disturbing and may be the reason for slow growth of the coral skeleton.

Picture 2 – pH measurement results after 45 minutes of brine mixing.
The chart shows that all tested salts gave solutions with a pH of 8 or above. This is good because even a larger one-off change will not disturb the pH of the aquarium. Especially in the mature tank where the pH level will quickly align.
Marine salt – KH after 45 minutes of mixing
At the end of the mixing, the KH measurement was performed using a Hanna Instruments HI755 pocket photometer. During the whole test, the KH measurement was performed twice. Once after 45 minutes of brine mixing and then by the MarinLab lab. The time period between measurements was about 10 days. Triton does not offer any KH measurements. KH results will be discussed below.
Marine salts – clarity
At the end of mixing, a black-and-white contrasting stencil was inserted into the bottom (18cm) of the bucket. The subjective assessment of clarity was made on the basis of turbidity of the water over the stencil. All salt solutions were evaluated in the same place and with the lighting as uniform as possible.
I have to admit that all marine salts with only one exception performed perfectly. Within 45 minutes of mixing all but one salts have clarified to satisfactory results. Only Kent solution of salt remained milky – even after 24 hours. A few years ago I had an encounter with Kent salt, but I do not recall such opacity. Turbidity often arises as a result of strong salt dampness when the reaction between calcium ions and carbonate ions occurs on the surface of neighbouring crystals. Colloidal calcium carbonate is formed, which is hardly soluble. Salt, however, was loose and looked dry. I suspect I might have gotten a defective copy.
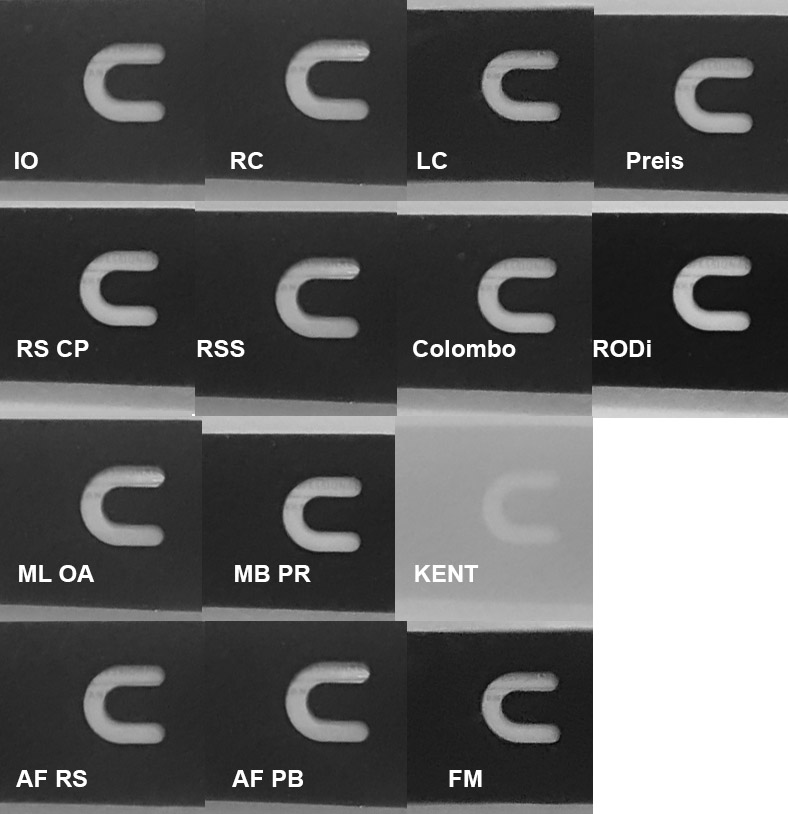
Picture 3 – Pictures of a stencil lying on the bottom of a bucket with mixed brine.
Marine salts – hygroscopicity
As it is known, salts tend to absorb moisture from the air (hygroscopicity). I have weight 100 grams of each salt and put them in separate containers. Then after 20 hours I weighed them again. The more hygroscopic the salt, the more moisture it catches from the air thus weighs more. We have to focus on couple variables.
First, heavier salt has reduced yield. We can suppose that the longer we use a pack, the more effectiveness it loses.
Secondly, the rate of absorption of moisture from the air depends on many factors such as opening frequency, air humidity, temperature fluctuations etc. And I expect it to be faster at the beginning of each package than at the end. Separate studies on this subject could be useful. Finally, too much salt dampness can make it unusable due to chemical reactions between its components
During measurement, the air temperature was around 21.5C and the relative humidity was at 46%.

Picture 4 – 100g samples of each salt absorbed humidity for 20 hours.
Let’s see how does it look on the graph:
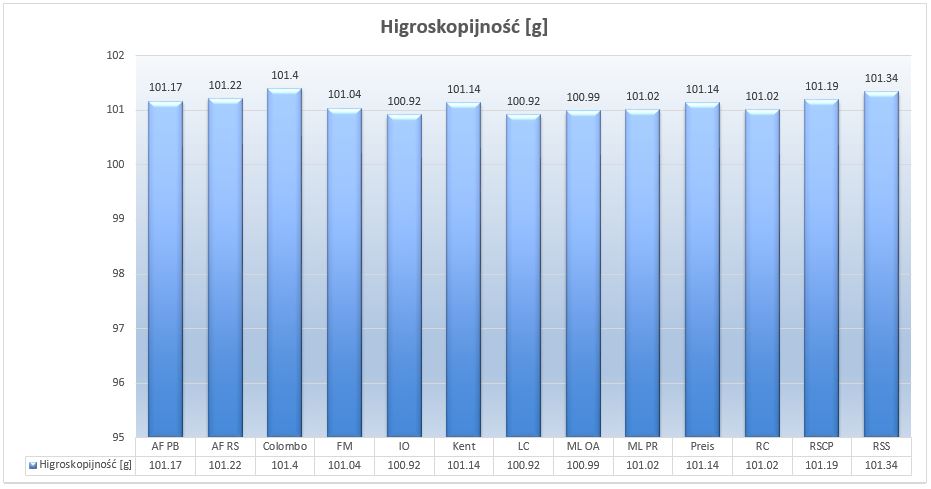
Picture 5 – Weight gain of 100 gram samples after 20 hours.
All salts performed very similarly giving a weight gain of about 1%. Living Colors and Instant Ocean gave the best results; Colombo the worst. Remember, however, that there is no poor quality salt here. Hygroscopicity is an inherent feature of salts, and the results obtained do not differ from those expected. The maximum weight range between the least and the most hygroscopic salt is 0.48g per 100g. Putting it on a 20kg bag we expect the loss of about 96g, that is the amount needed to salivate about 2.3L of water at 35ppt. To sum up, the average salt loses about 1.12% of its effectiveness on the packaging.
Marine salts – ability to agglomerate
The unfortunate feature of salt, which results directly from hygroscopicity is the ability to agglomerate. Neighboring crystals connect their crystalline lattices to form lumps, which in extreme cases can retain the shape of the container in which they are located. This is, of course, an unfavorable feature that can lead to salt being useless. If the lumps of salt are easily breakable, such salt can be used further. However, if the contents of the bucket are stone hard, such salt is usually thrown away (or complaint).
After hygroscopic examination, all salts were gently removed from the containers and aligned with each other for comparison.

Picture 6 – After weighing, slats were gently removed from their containers
We can clearly see that 3 marine salts have preserved the container shape. These are: Kent, Reef Crystals and Instant Ocean salts. Red Sea Coral Pro and Living Colors were loose the most.

Picture 7 – Salts after a gentle crush.
Then, with a little force, I tried to crush the “salt pucks” to see how fossilized they are. Basically, apart from Instant Ocean and Kent, they all fell apart. I will add that eventually each of the salt has surrendered, although Instant Ocean, Reef Crystals and Kent, despite being loose, seemed damp.
Aquarium salts – Element analysis
To test the chemical composition, I took samples of water from each batch and sent it to two different laboratories for an ICP-OES spectrophotometer test. One sample was sent to German Triton and the other to Polish MarinLab. I know, I know … I can already hear the voices of indignation … because it is well known that Aquaforest and MarinLab have the same owner. However, I decided to do this for several reasons. Firstly because the MarinLab’s ICP measurement was not the only measurement with the spectrophotometer, and with the I can easily verify them results of the Triton lab. Secondly, all the samples were sent to the lab anonymously and only I knew which number corresponds to which salt. Finally, this situation gave an incredible opportunity to check the reliability of the results from both laboratories and to compare them directly.
While at the ICP topic, I will mention how it is done, so you will see the whole picture while reading graphs down below. Although the ICP-OES is currently the highest quality analysis method available for marine aquarists, it has certain problems which can impact the accuracy and precision of the seawater results especially with smaller ranges of trace elements. For macroelements, a single flow process (single pass) gives you a fairly good results of 3-5% accuracy (https://reefs.com/magazine/triton-lab-icp-oes-water-testing-154/) and from what I know, MarinLab makes two separate measurements – for micro and macroelements. I do not know what analytical and standardization procedures Triton and MarinLab implemented, but for the test sake I will accept +/- 4% discrepancies between results from the two companies. On the other hand, it is an analysis of complex solutions with unknown parameters, so there is no way to undermine or deny any result.
The significant difference between the Triton and MarinLab tests is that, for a small extra, MarinLab also makes it possible to carry out an RODi water test. This allows you to check the quality of the reverse osmosis system without buying an additional test. RODi water test may be the background to subtract from the aquarium water test results. For Triton, a separate test is required.
The results will be related to the marine water composition given by Karl K Turekian in his 1968 work “Oceans.” These studies may not be the latest because they were published nearly 50 years ago, but for our needs they are sufficient. Another thing is, the quantitative composition of the seawater depends on the salinity of the water and changes (keeping the relationship between macroelements – Diettmar Rule) from 32ppt in the Alaskan Gulf area to 40ppt in the Red Sea. And even if we only consider tropical regions, the salinity will vary from 34 to 40ppt. Therefore, in order to partially facilitate the analysis, I will assume that the basis for comparison will be the salinity of 35ppt (K. Turekian’s work), and the results of – 3% to + 14.3% will be optimal for aquarium inhabitants. The optimum will be marked with two horizontal lines. I know this is not the best way to do this, but otherwise only NSW will have the perfect parameters and every test salt will lose to it.
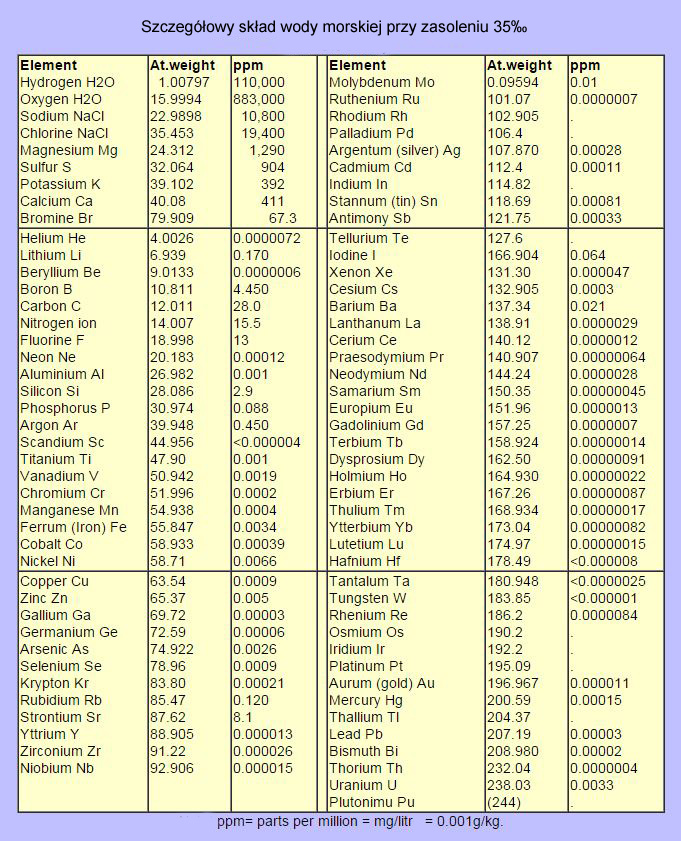
Picture 8 – Average chemical composition of seawater at salinity of 35ppt (source: Karl K. Turekian Oceans, 1968)
Marine salts – Calcium Ca
Calcium, together with carbonate ions, forms the basis for coral reefs. It is thanks to them coral skeletons shell molluscs or other limestone structures are created. It is not surprising that aquarists require their sea salt to have adequate levels of this element. NWM contains an average of 411mg / L.
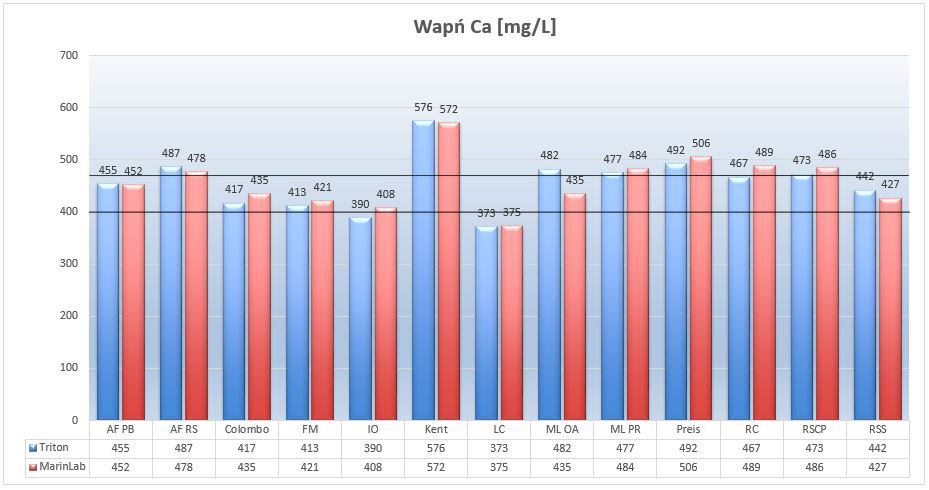
Picture 9 – Calcium Ca (mg/L) in tested salts at salinity of 35ppt.
The vast majority of the examined salt had results above natural values. Fortunately, calcium is one of those elements to which corals are quite tolerant. From my experience I know that, in the aquarium, SPS corals feel good at the levels of this element in the range of 380 – 480mg / L. AF PB, Colombo, FM, IO and RSS salts meet this premise. Kent Salt showed the highest results, much above the optimum, and Living Colors Salt was the only salt with little calcium deficiency relative to the optimum.
Except for one salt, all results from both labs correspond to each other, assuming a margin of +/- 4% for error.
Marine salts – Magnesium Mg
Magnesium plays a significant role in the balance between calcium and carbonate in the seawater, which is overrun by these ions. Magnesium blocks the precipitation of calcium carbonate in water. Low levels of magnesium cause calcium and carbonate to “escape” from the solution.
In the NSW the level of magnesium ions reaches about 1290mg/L at the 35ppt salinity. As a fun fact I will add that in reef in areas with higher salinity (Red Sea, Kuwait Bay) magnesium can be even above 1700mg/L (source: Warehouse – water and cleanup, January 2005). However, we will return to our optimum of -3% to + 14.3%.
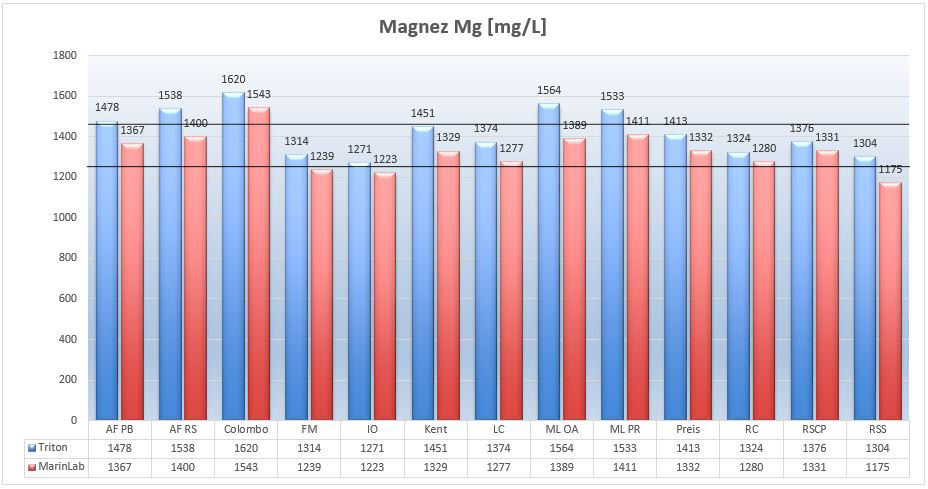
Picture 10 – Magnesium Mg (mg/L) in tested salts at salinity of 35ppt..
Except for the Colombo salt, all tested salts achieved at least one result in the given optimum. The most similar to NSW were Fauna Marin (FM), Living Colors (LC), Instant Ocean (IO) and Red Sea Salt (RSS) salts. Significant excess of magnesium levels was detected in Colombo salts. Fortunately, these are not dangerous levels.
As far as comparing ICP-OES results, you can see that all MarinLab results are lower than those from Triton. The four salts results: AFRS, AFPB, MLOA and MLPR from both laboratories have achieved greater variance than previously set +/- 4%
Marine salts – Alkalinity KH
Alkalinity (KH) is one of the most important water parameters in a marine aquarium. Holding KH at an appropriate level has an impact on a range of processes, from controlling pH fluctuations, through probiotic filtration efficiency, to calcification efficiency of limestone. The parameter itself is quite complex and often misunderstood. For more information on alkalinity (KH) in the aquarium visit: http://reefhub.pl/kh-czy-alkalicznosc-o-c-w-tym-chodzi/ Fortunately for us aquarists, alkalinity (KH) translates in a simple way into the amount of bicarbonate ions in water. They are the source of carbonates during the calcification of CaCO3 calcium carbonate.
Let’s see the results on the graph.
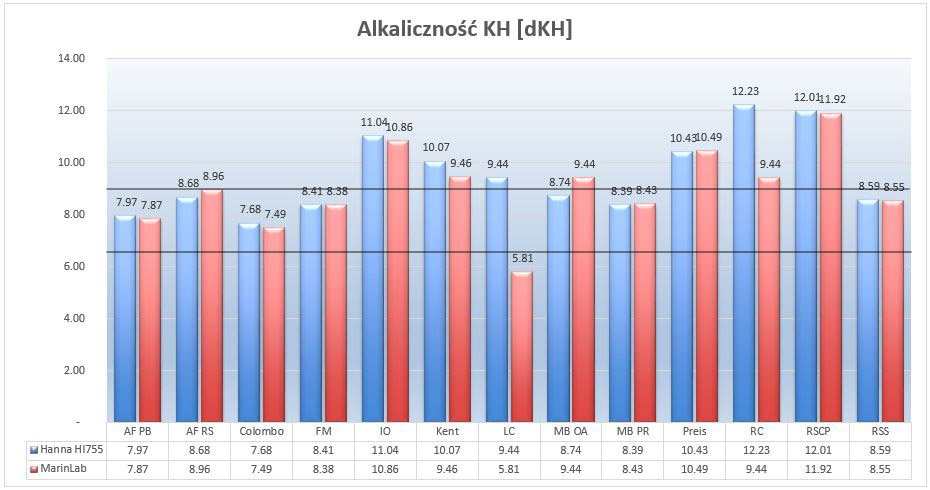
Picture 11- Alkalinity of the tested brines at 35ppt. Blue color – results marked with Hanna HI755 photometer after 45 minutes of brine mixing. Red color – results from MarinLab about 10 days after mixing the brine
Aquarium salt is a chemical base in the aquarium and, together with various methods of macro and micronutrient supplementation, forms the basis of the chemical environment in the aquarium. Many times in different forums I said, that in my opinion, in order to create domestic reefs we have to copy what nature gives us – that is, ready NSW parameters on the reefs. Alkalinity of seawater is about 7dKH and unfortunately, none of the salts has reached this level (assuming that the MarinLab’s LC result is a mistake).
In my aquarium, KH stays at 7dKH level and I replace 5% of water per week. Striving for the stability of this parameter, I can assume that for my needs salts with KH less than 9dKH will be suitable. Then the change of KH during the water change will be about 0.1dKH (9 – 7×5% = 0.1), which should not irritate the corals. On this basis, the optimum range is 6.5 – 9dKH, and the salts within this range are both AF, Colombo, FM and both Microbe – Lift salts
What about salts with higher alkalinity? I wrote about it in the previous test mentioning Red Sea Coral Pro, which is famous for its high KH. For details, I refer to the previous test.
The next thing that caught my eye was the similarity between the results of both measurements. I do not know the method of marking KH by MarinLab, but I get two conclusions. Firstly, the KH level in the closed tube is relatively stable over time, and second, I have another proof that the HI755 photometer gives reliable results.
Two salts are an exception – Living Colors and Reef Crystals. In both cases, the result from MarinLab is much lower than in one using Hanna. There can be many reasons, so I do not even try to guess. I will just add that in the previous KH test of the Reef Crystals salt after 45 minutes of mixing resulted in 12.5 dKH
Marine salts – Sodium Na
Sodium and chloride ions are considered as the main regulators of salinity. In our study, however, we do not have the results of chloride concentrations, which makes it more difficult to interpret the results.
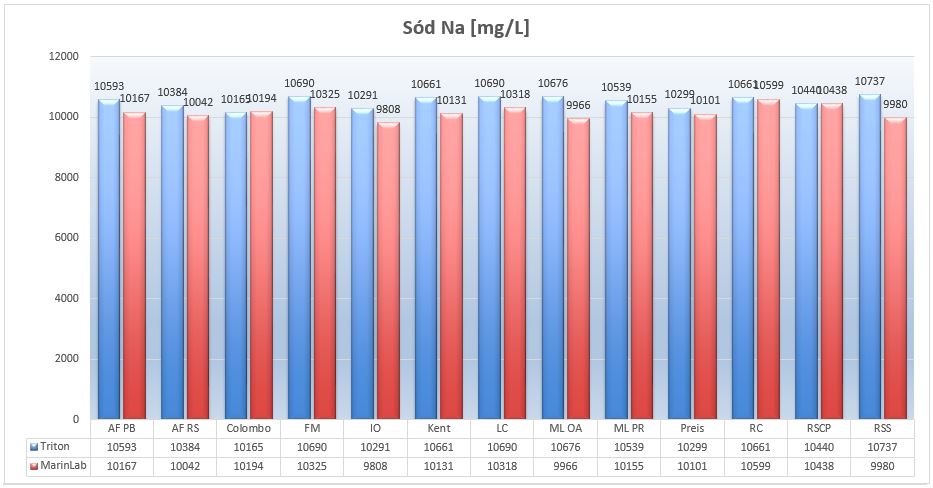
Picture 12 – Sodium Na (mg/L) in tested salts at salinity of 35ppt..
Sodium in the seas at salinity of 35ppts reaches 10800mg/L. The graph shows that all salts have a similar levels of sodium ions.
The results from both ICP-OESs correspond to the accepted error of +/- 4%.
Marine salts – Potassium K
Potassium ions are important for coral metabolism. Here I wrote more about potassium: http://reefhub.pl/potas-suplementacja-w-akwarium-morskim/. Let’s see the potassium levels detected in the tested salts:
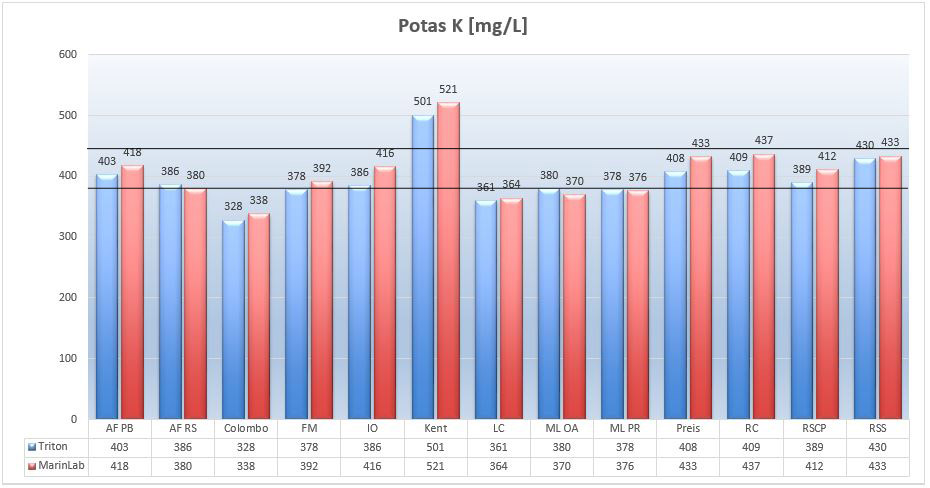
Picture 13 – Potassium K ions (mg/L) in tested salts at salinity of 35ppt.
The potassium level in NSW is about 400mg/L (Turekian gives 392mg/L in its work). The graph shows that most of the tested marine salts have a well-balanced potassium levels of 380-450mg/L. The Kent salt is an exception with a potassium level of over 510 mg/L, and Colombo salt with levels of 340 mg/L.
Results from the Triton and MarinLab labs correspond to the assumed error margin of +/- 4%
Marine salts – Bromides Br
The level of bromide ions in the NSW is about 67 mg/L. Unfortunately, scientists have different opinions of the role of this element in coral metabolism.
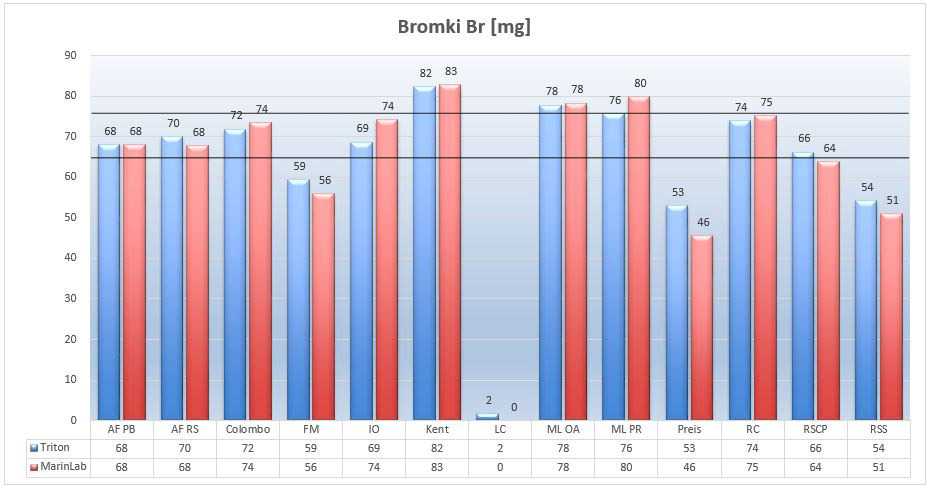
Picture 14 – Bromide Br ions (mg/L) in tested salts at salinity of 35ppt.
As we can see from the the chart, the greatest excess of bromides relative to the NSW came out in Kent salts. The zero of bromide in Living Colors indicates that the producer intentionally omitted this element while balancing his salt. Not so long ago, AFPB salt also did not have bromide in its composition. The manufacturer rightly explained that bromide-free salt could be used in systems with ozone generators (bromides treated with ozone, oxidized to harmful bromates). I wonder if the LC salt producer justifies the lack of bromides in their salt with the same explanation.
Except for the Preis salt, the results of the both labs correspond to each other in the accepted range.
Marine salts – Boron B
In general, boron is not an element that needs to be carefully controlled. Scientists say that boron can be an essential nutrient for some marine organisms, but also that it can be toxic to others with little over natural levels. It is recommended to maintain its NSW level of around 4.4mg/L, although levels below 10mg/L are generally tolerated. (Http://reefkeeping.com/issues/2004-05/rhf/) The chart below shows that all tested salts have fulfilled this requirement.
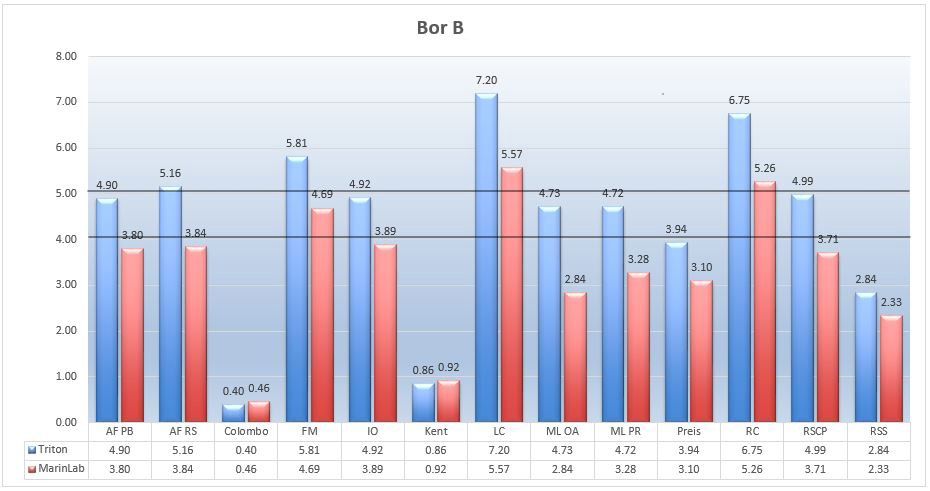
Picture 15 – Boron B (mg/L) in tested salts at salinity of 35ppt.
Triton and MarinLab spectrophotometers results vary, making it difficult to identify the best salt, but by average, we find that both the Aquaforest, Instant Ocean and Red Sea Coral Pro salts are within a preset optimum of boron content of 4.2-5mg/L. The other salts if they are not too far from optimal values do not show dangerous amount of boron. Producers Colombo and Kent have determined that their salts will have only minimal amounts of this element.
Marine salts – Strontium Sr
The level of strontium in the NSW according to Turekian is 8.1 mg/L, and the recommended level of this element in the aquarium should be within 5-15mg/L (http://reefkeeping.com/issues/2004-05/rhf/). Higher levels of strontium ions may be toxic to some animals
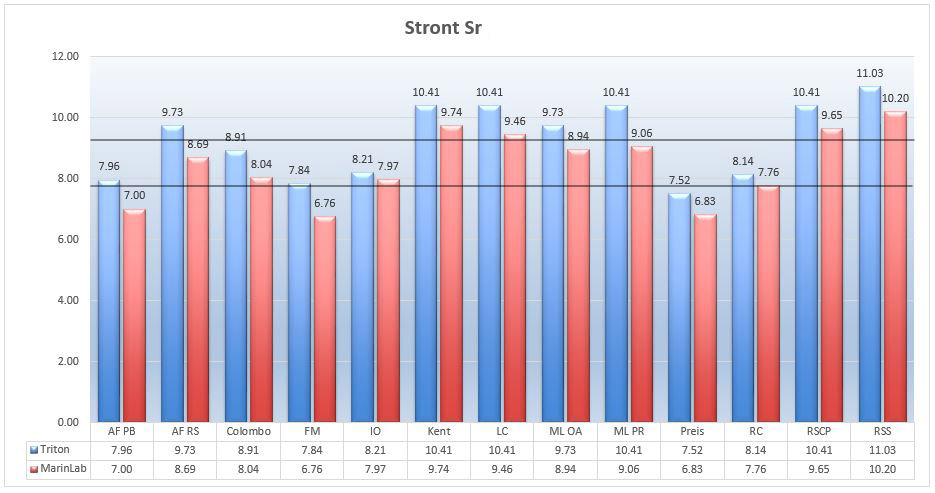
Picture 16 -Strontium Sr ions (mg/L) in tested salts at salinity of 35ppt.
The content of strontium ions in any salt does not raise any objections. All the results showed its level near the optimum.
As for the comparison of the results, you can see again the tendency in which Triton has higher results than those from MarinLab. The results are comparable, although most differences between the results are above +/- 4%
Marine salts – Sulfur S
In the NSW the level of sulfate is about 2700mg/L and the sulfur level is about 900mg/L. Looking at the molar mass of sulfates (96g/mol) and sulfur (32g/mol) it is easy to see that almost all sulfur in NSW comes from sulfates. Its levels are not, however, particularly important for marine organisms. We may find here the relation to the sulfur cycle in nature and its different levels of oxidation by facultative bacteria in the DSB SO4<>S deposit. This is not something we have to control. Older versions of Balling’s methods have suggested the use of magnesium sulphate (MgSO4) but this could lead to sulphate accumulation in a system where its consumption is very low.
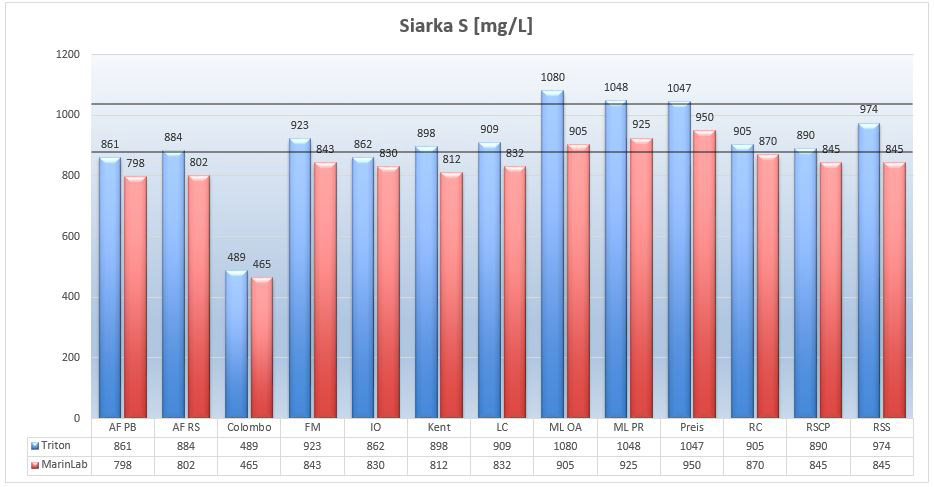
Picture 17 – Sulfur S (mg/L) in tested satls at salinity of 35ppt.
In most tested salts the sulfur (sulfate) results oscillate near the NSW optimum. The exception is the Colombo salt, where sulfur (sulphate) levels have been reduced by about 50%. This is not alarming, as the consumption of sulfate in the aquarium is negligible.
The results from both labs are similar, with the exception of Microbelift salts and Red Sea Salt. Again Triton’s results are higher than MarinLab’s.
Marine salts – Silicon Si
To start, we will deal with silicon, which is often blamed for the problem of diatom plague. We have to keep in mind that the ICP method shows total silicon from different compounds, whether they are biologically active or not. The silicon in ICP results may come from silica SiO2, which is a component of sand or glass. This is a generalization, but this type of silicon compounds is harmless to us, just as dangerous as sand or glass in an aquarium. It is enough, however, that sand dust will float in the water, and ICP will show elevated levels of silicon.
Different situation is with SiO4 silicates. These compounds, as soluble, may be the direct cause of diatom blooms. Unfortunately, in our study we are not able to determine these compounds, so we need to approach the results carefully. However diatoms are important organisms from a biological point of view. It is estimated that 24% of the natural production of oxygen and 25% of organic matter in the oceans comes from diatoms (Wikipedia).
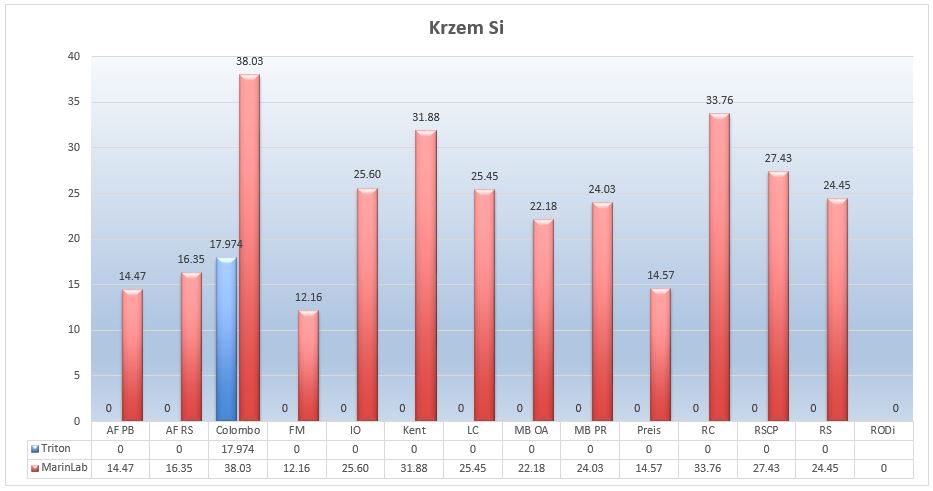
Picture 18 – Silicone Si (µg/L) in tested salts an salinity of 35ppt
In the NSW the level of silicon is about 2.9mg/L. All of the salts showed levels of silicon Si much below NSW. The highest level of silicon was detected in Colombo salts – 38μg/L, which is about 76 times lower than in seawater.
Trace and toxic elements
Next we will look at a group of elements commonly referred to as microelements. Some of them are biologically important from the supplementation point of view, and some, because of their toxicity, are highly undesirable in water.
Let’s take a look at the table below. All the results are referred to the table with the composition of seawater. Since most of the trace elements (vanadium, zinc, nickel, etc.) are not necessary for supplementation in marine aquariums, and those which are necessary (iodine, iron) can be relatively easily dosed. That’s why all the results below the NSW levels were marked in green, all over but under 14.3% in orange and above 14.3% in red. On the right side I gave the RO test results used to produce brines and the level of the element in NSW.
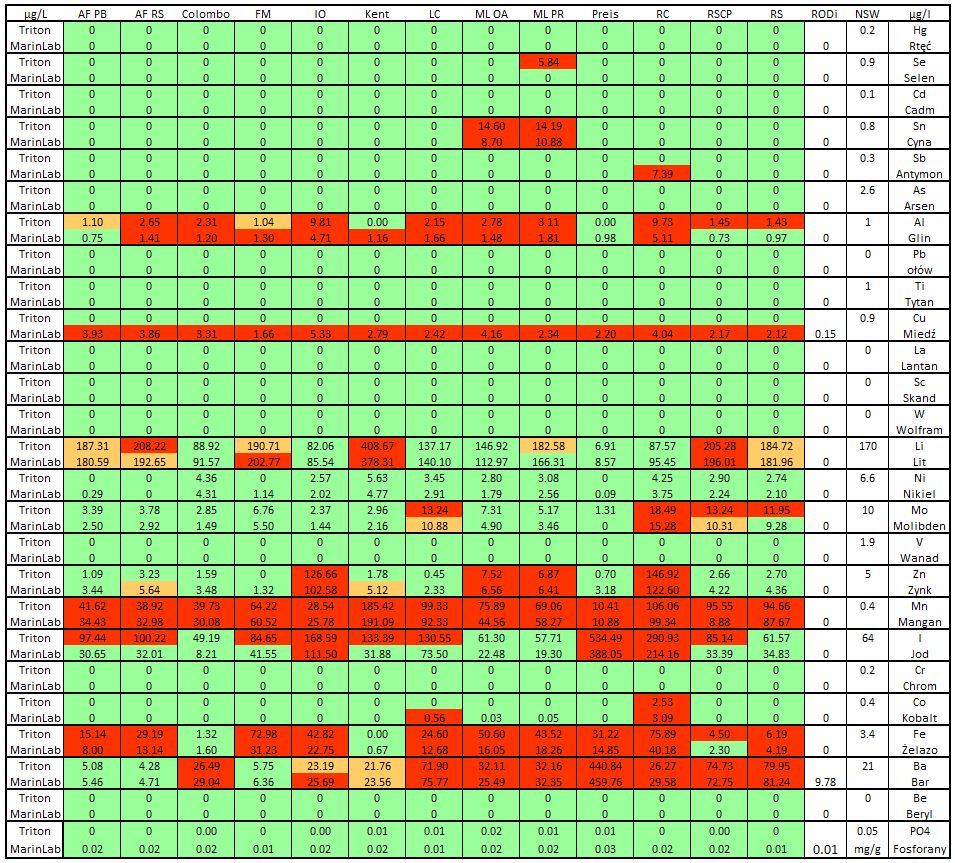
Picture 19 – Summary table of trace elements and phosphates. All the results below the NSW levels are marked in green, all over the NSW level but under 14.3% in orange and above 14.3% in red
Looking at the results above, we need to analyze them through the potential imperfections in the ICP-OES method while marking all components of seawater. We also need to keep in mind that we have results from two different laboratories, probably using different devices and calibration procedures.
It is perfectly evident in the case of silicon (Figure 18) and copper, which, according to Triton, is not present in any of the saline solutions tested, and according to MarinLab, is found in all – even in my RODi water. This would suggest another detection threshold (LOD) or conservative measurements in both laboratories. I have no way of saying which results are correct. Probably the truth lies somewhere in the middle.
On the other hand, many of the trace elements in salts may be present as contaminants of major constituents rather than as deliberate supplementation. In this situation, salts with higher macroelement concentrations may show the presence of some micronutrients.
My remarks:
The single results of selenium in ML PR salt (Triton) and antimony in RC salt (MarinLab) – personally I would not be bothered with them.
Microbe-Lift salt is the only one to have exceeded the tin confirmed by both laboratories.
Except for the Preis and AF PB salts, in all other salts, at least one result of Aluminum Al is outside the optimal level of NSW
Lithium is most exceeded in Kent salt. The remaining salts hold optimum lithium levels or slightly exceeded them (RSCP)
Big amount of excess zinc was detected in the French IO and RC salts
All tested salts showed significant levels of manganese surplus. The best one here is the Preis salt. The interesting fact is that in the previous test AFRS salt was the only salt with zero levels of manganese.
Iodine almost halved the results. Most of Triton’s results indicated that this element exceeded ten of the 13 salts tested. MarinLab pointed out only three. The RC, IO and Preis salts were the worst, with the highest exceedance.
Iron also colored the table in red. The highest exceedances were detected in FM and RC salts. In Colombo, Kent and RSCP (according to MarinLab) salts, this element does not show any excess levels.
Results showed excess barium in eight of the tested salts – in Preis salt, twenty-one times over the NSW level. The next two were at the edge of the optimum, both Aquaforest and Fauna Marin salts had appropriate levels of this element. The interesting fact is that MarinLab detected small amount of barium in the RODi water. The problem was that it was higher than later detected in AF and FM salts.
Phosphate levels in the NSW depend on many factors such as season, depth, currents, distance from the estuary. E.Borneman in his book gives the average PO4 concentration at 0.13 mg/L. In other studies, I have found information on phosphates in the Philippines that oscillate between 0.001 and 0.08mg/L. Due to the fact that we generally aim for the lowest PO4 level in water, I have adopted a reference level of 0.05mg/L (http://pubs.sciepub.com/jas/4/1/1/). All tested salts meet this requirement, with MarinLab’s ICP again being more sensitive to the tested parameter than ICP from Triton.
Sea salt – Summary
Since marine aquaculture has become a popular hobby, aquarists can choose from many brands. Although the test above does not consider all the salts available on the Polish market, it gives an interesting picture of the thirteen selected for the test. First of all, the results are a confirmation of the general opinion that there is no perfect salt. Some are better at these parameters, others in different ones. This would suggest that a good idea would be to change the salt after several packs.
By taking this test, I had to accept certain assessment and reference criteria. I decided that it would be best to compare the brine to NSW. This does not mean, however, that the increased results of some parameters disqualify the given salt, because on the Internet you can find beautiful tanks with each of the tested salt.
Before I go into the summary of individual salts, I would like to once again thank all those who helped with this project and the commercial sponsors. Thanks to them this test was possible. For all readers of this article, consider yourself invited to shop in the following stores:


And for all the shops, in the following wholesalers:
The above test was conducted ad hoc and although I carried it out thoroughly, it is not a typical test. I hope, however, that the information collected will be useful to you and will help you make the decision when buying salt.
Aquaforest AFPB – Salt of the Polish producer, which became very popular on the Polish market. It fails a little in terms of performance, but compensates for the fact that chemically it is probably one of the most refined of all tested salts. By averaging ICP results from both laboratories there are no disturbing surges, and in the trace elements table this salt falls as one of the better ones. Aquaforest Probiotic Salt is advertised as a salt containing probiotic bacteria and their nutrient.
Aquaforest AFRS – the second salt of our producer, differing mainly in that it does not contain any probiotics. In terms of composition of trace elements, it was slightly worse than its twin. From the macroelements point of view it is a bit richer. This is one of the salts I also tested in the previously. It is clear that the salt composition has changed. This is illustrated by the sulfur content, Strontium Sr, and Mangan Mn. In the previous test, this was the only salt in which no trace of this element was detected. As you can see today, all the salts tested have clearly too much of this element.
Colombo – is a relatively new on the Polish market. With optimal KH and Ca, a lot of magnesium would suggest that this salt is ideal for calcium reactors because many calcium media suffer from deficiency of this element. The manufacturer decided to lower the sulphate level to the NSW and almost completely eliminated Boron B. This is the only salt in which both ICPs showed silicon Si content (well below NSW level), but the test itself is not able to show whether it comes from undesired silicon compounds. This salt came out very well in the micronutrient table and is one of the two tested salts that have shown very low levels of iron. This allows the salt to be particularly good for the yellow SPS coral colour.
Fauna Marin – FM – German producer’s salt generally regarded as very good but too expensive on our market. It has good performance and almost perfectly fits into the macroelements optima. It also has the lowest level of silicon Si. Table of micronutrients shows that it is quite pure salt, however, has one of the largest iron Fe exceedances among all of them.
Instant Ocean – IO – is a French salt of quite good reputation in Poland. Historically it is considered as a poorer salt, recommended for less demanding reef systems. Indeed most macronutrients results are at the lower side of the optimum. In the table of micronutrients with high levels of iodine I and Zn zinc, it ranks in the lower half of tested salts. Very good performance, however, has a very high tendency to agglomerate under the influence of moisture. While using this salt, it is important to remember to seal the bucket tightly. It’s strange that the manufacturer does not pack the salt in an extra bag just pouring it loosely into the bucket.
Kent – known for years on the Polish salt market, though not particularly popular. In the study, the biggest let down was the lack of clarity and lottery when it comes to composition of macronutrients and micronutrients. On the one hand, high levels of calcium Ca and potassium K, and on the other hand, almost no boron B. With the largest exceedances of lithium Li and manganese Mn and near almost undetectable traces of iron Fe shows a mess in the balance of salt. There is also great susceptibility to agglomerate. Maybe it’s just an unlucky series, but the results give you some idea.
Living Colors – LC – is a relatively new but popular salt on the Polish market. The macro results qualify this salt rather to the poorer ones, because most of them are located near the bottom of the optimum. Only boron B exceeded. This is the only salt with practically none bromides, which is recommended for systems with intensive ozone. It is a salt that is perfectly loose and not susceptible to agglomerate. In this aspect, it was probably the best of the tested salts.
Microbe Lift Organic Active – MBOA – Analysis of macroelements of this salt came out very well with most parameters lying around the optimum. It is a little worse in terms of micronutrients. Both Microbe Lift salts were the only ones showing tin contamination with tin Sn and slight zinc Zn excess. Unfortunately I was not able to find any information what bioactive ingredients hide under the “Organic Active”
Microbe Lift Premium Salt – MBPR – is the second salt from the Microbe Lift stable. The word “twin” ideally fits here, because the results of both salts are very similar. Both macro and microelements. Triton has detected Se selenium in this salt, but I suspect this is a measurement error. Wonder why the manufacturer decided to release two lines of salt, since both came out similarly? It looks like the difference really lies in the magical “Organic Active” phrase. Either way, both Microbe Lift salts have shown themselves as very interesting
The results of the Preis salt showed quite high levels of KH and Ca, whereas the deficiency of Bromides Br and Boron B. The salt showed one of the lowest Si silicon levels but had the highest concentrations of iodine I and barium Ba of all tested salts, among which it had the lowest manganese Mn and Aluminium Al contamination. Salt showed considerable resistance to agglomeration.
Reef Crystals – RC – is of the same manufacturer as Instant Ocean. In Poland it is considered as richer. Indeed, most macroelement results show higher values than IO salt. RC salt also presents numerous micronutrient levels. It tends to agglomerate, but even then, it’s quite easy to crumble.
Red Sea Coral Pro – RSCP – is a salt with historically high levels of Ca, Mg and KH, but while calcium and magnesium lie in the optimum area, KH of RSCP salt reaches the highest levels among tested. This is part of the Red Sea program, but those planning to switch to this salt should take into account its gradual implementation. Salt exhibits good resistance to agglomeration. In the table of micronutrients, it turned out quite decent. Despite some red fields, most of them are small breaches.
Red Sea Salt – RSS- is Red Sea’s twin salt, due to the color of the bucket often referred to as the Red Sea Blue (as opposed to the black salt bucket RSCP). Most macronutrients results are in perfect fit. The results of trace elements do not differ too much from its twin. It is worth mentioning that both Red Sea salts achieved the best performance in grams at 35ppt.





